
In response to the growing demand for
pharmaceutical-grade polyvinyl alcohol (PVA),
we built a new dedicated plant
that has been operational since 2023.
The product is now marketed
under the brand name “POVASEAL.”
Product Lineup
-

Fine Powder Type
Fine powder type with good mixability for powders, including active pharmaceutical ingredients (APIs) and inorganic substances.
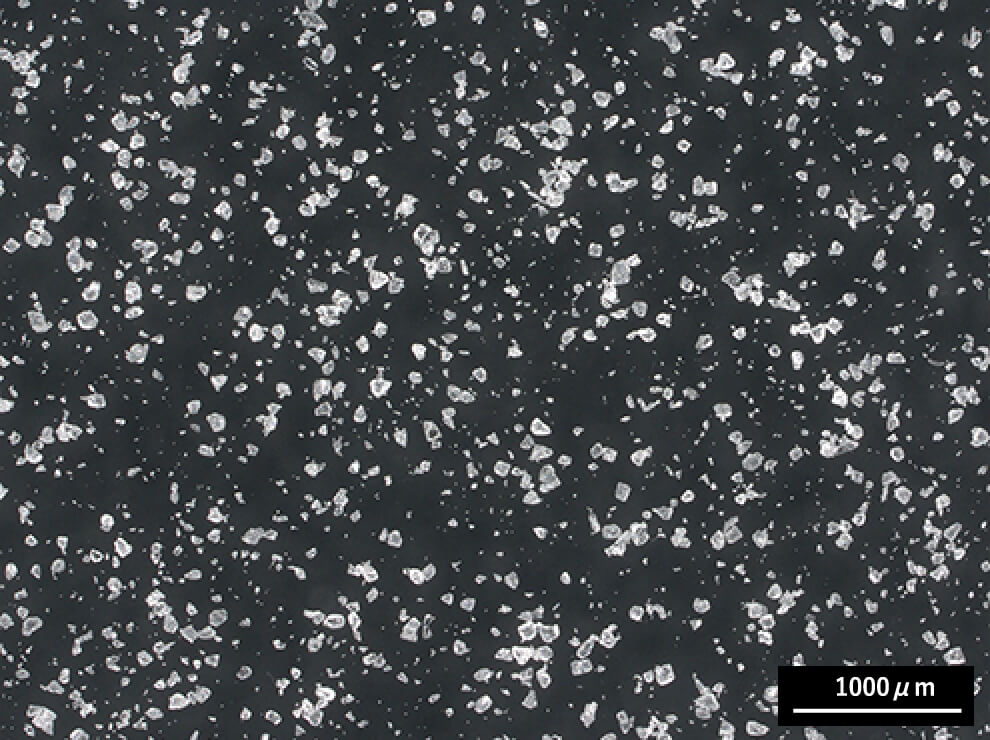
-

Granule Type
Reduced tendency to form lumps when added to water. Suitable for heated dissolution processes.

Both are low-degree-of-polymerization, partially hydrolyzed PVA products,
differing only in particle size.
Quality Specifications
|
POVASEAL 05PS (Fine Powder Type) |
POVASEAL 05P (Granule Type) |
|
|---|---|---|
| 4% Viscosity (mPa•s) | 4.6 - 6.0 | 4.6 - 6.0 |
| Degree of Hydrolysis (mol%) | 86.5 - 89.0 | 86.5 - 89.0 |
| * Particle Size | D50: 60 - 70 µm | Approx. 100 - 1200 µm |
| Previous product name | PE-05JPS | PE-05JP |
* Note: Particle size is a target value, not a specification.
Compliance with Pharmacopoeias
Polyvinyl alcohol (PVA) is listed in major pharmacopoeias.
POVASEAL meets the specifications of the following pharmacopoeias.
| Pharmacopoeia | Listed Name | |
|---|---|---|
| Japan | Japanese Pharmaceutical Excipients (JPE) | Partially Hydrolyzed Polyvinyl Alcohol |
| United States | United States Pharmacopeia (USP) | Polyvinyl Alcohol |
| Europe | European Pharmacopoeia (EP) | POLY(VINYL ALCOHOL) |
| China |
Pharmacopoeia of the People's Republic of China (ChP) |
Polyvinyl Alcohol |
Regulatory Information
Self-Imposed Good Manufacturing Practice (GMP) Standards for
Pharmaceutical Excipients Certification Number: GAB0240055(POVASEAL)
US Drug Master File (DMF) No. 038552 (POVASEAL 05PS) US DMF No. 040206 (POVASEAL 05P)
Chinese DMF:
CDE No. F20190000286
Key Features of Pharmaceutical-Grade PVA “POVASEAL”
- ●Grinding, homogenization, and packaging are performed using equipment compliant with the Self-Imposed GMP Standards for Pharmaceutical Excipients (Hardware).
- ●Manufacturing management and quality control follow procedures based on the Self-Imposed GMP Standards for Pharmaceutical Excipients (Software).
- ●Certificates of Analysis (CoA) conforming to the Japanese Pharmaceutical Excipients (JPE), or the United States Pharmacopeia (USP), or the European Pharmacopoeia (EP) are issued, guaranteeing that analytical values meet the requirements of each pharmacopoeia.
- ●The product is packaged in 20 kg kraft paper bags with polyethylene inner liners. Both inner bag and the outer packaging bag are heat-sealed to prevent foreign matter contamination. The outer bag features an easy-open tape for convenient removal of the inner bag.
Functions and Applications of PVA
Polyvinyl alcohol (PVA) is a water-soluble synthetic polymer with versatile properties. In pharmaceutical formulations, it serves as a binder in wet granulation and as a film-coating agent for tablets. It is also used as a carrier in solid dispersions to enhance the solubility of poorly water-soluble active pharmaceutical ingredients (APIs).
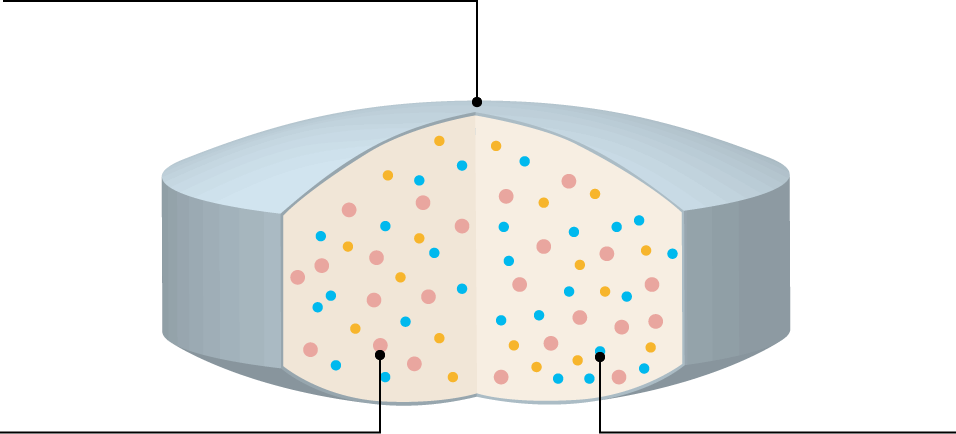



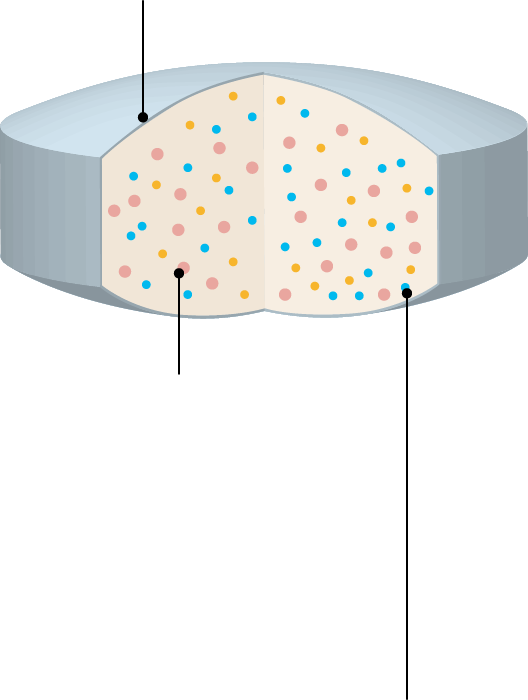



| Uses | Functions of PVA | Expected Effects |
|---|---|---|
| Coating |
Film-forming properties Flexibility of the film Gas barrier properties Moisture barrier properties |
Prevention of API oxidation and moisture absorption prevention Prevention of tablet cracking |
| Binder | Adhesion and bonding ability |
High tablet hardness Low friability Prevention of capping |
| Solid Dispersion | Emulsification and dispersibility |
Improvement of bioavailability of poorly water-soluble APIs |
Technical documentation is available on the following topics:
Tablet film coating with PVA (moisture-barrier formulations)
Wet granulation using PVA as a binder (high-shear mixer granulation and fluid bed granulation)
Please contact us for more information.

