Overview
Vinyl carboxylate is a substance in which a vinyl group has been introduced as an ester to a carboxylic acid, an example of which is acetic acid.
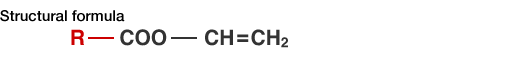
For example, take vinyl acetate (VAM), one of JVP’s main products, and use a carboxylic acid as an ingredient in place of acetic acid to produce a vinyl carboxylate that corresponds to the ingredient acid. Or also, butyric acid can be used to make vinyl butyrate or lauric acid can be used to make vinyl laurate.
The vinyl carboxylate formation method developed by JVP is a superior method because it uses no mercury catalyst, which is used in conventional methods, and can produce products with a high degree of purity.
JVP offers a lineup featuring many types of vinyl carboxylate.
Please refer to the Product List for details
Applications
Currently, there are no other vinyl carboxylates that are being used in such large quantities as vinyl acetate, but because of the diversified structure of these substances, their possibilities are limitless. Following is an introduction to only a fraction of the applications for vinyl carboxylates.
- Controlling of plastic softness (Tg) and other properties (used as a copolymerization monomer)
- Using vinyl carboxylates with functional groups as cross-linking sites
Controlling of plastic softness (Tg) and other properties (used as a copolymerization monomer)
When synthesizing polymers, producing a product with just one type of monomer is not common. Instead, usually a variety of monomers are copolymerized to approach the desired properties such as the plastic’s hardness and solubility in solvents.
Vinyl carboxylates are very useful as this type of modification monomer.
For example, a polymer polymerized using only vinyl pivalate will produce a transparent, hard plastic (Tg: 86°C). Copolymerizing this type of monomer that produces a hard polymer makes it possible to produce a plastic that does not soften easily even at higher temperatures.
Conversely, a plastic with flowability at room temperature can be obtained by polymerizing a straight-chained aliphatic vinyl carboxylate like vinyl laurate. Copolymerizing this type of monomer that produces a “soft” polymer makes it possible to obtain a soft “internal plasticity” plastic without raising its temperature.
(Tg: Glass Transition Temperature): This is the temperature at which a polymer changes from a hard glass-like state to a soft state.
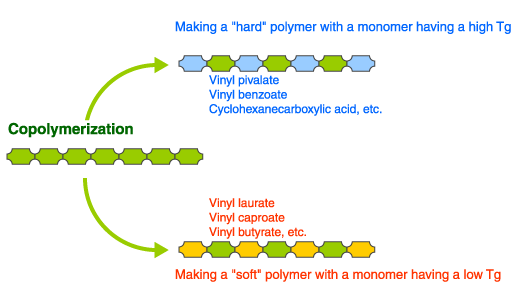
A plastic’s properties can be modified in other cases using copolymerization to obtain specified objectives, such as controlling the solubility in solvents, increasing the dispersability of pigments, or adding cross-links.
Using vinyl carboxylates with functional groups as cross-linking sites
Example: Acrylic rubber cross-link site monomer
Vinyl monochloroacetate has an active chlorine atom, so the reactivity of this chlorine atom can be used to cause a reaction.
The following is an example of using this active chlorine atom as an acrylic rubber cross-link site (area that reacts with a cross-linking agent).
Acrylic rubber is a rubber with excellent heat resistance and oil resistance and is mainly used as a material in parts that require both resistance to high temperatures and oil, such as for parts used in the automobile engine compartments.
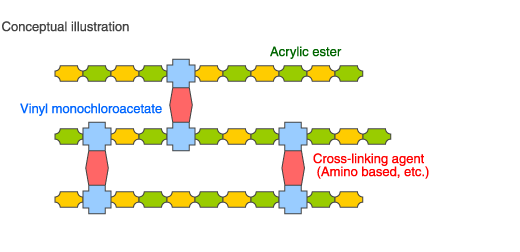
A few percent of vinyl monochloroacetate (shown in blue in the illustration) is copolymerized with various acrylic ester monomers.
Using an amino based or other cross-linking agent here will quickly form cross-links. Since the chlorine atom that forms the leaving group of the vinyl monochloroacetate is very active, the formation of the cross-link is very fast compared to when using 2-chloroethylvinylether, which is also a chlorine base vinyl monomer, and secondary vulcanization is in some cases unnecessary.
Product List
| Structural formula | CAS No. | Melting Point (˚C) | Boiling Point (˚C/mmHg) |
Flash Point (˚C) | Specific Gravity (g/ml, (˚C)) |
Polymer Tg (˚C) | |
| Vinyl Acetate |
CH3COO-CH=CH2 |
108-05-4 | -96.5 | 72.7/760 | -5.0 | 0.9342(20) | 28 |
| Vinyl Pivalate | (CH3)3CCOO-CH=CH2 | 3377-92-2 | -82 | 111/760 | 14.5 | 0.8709(20) | 86 |
| Vinyl Monochloroacetate | Cl-CH2COO-CH=CH2 | 2549-51-1 | -34 | 136/760 | 51.0 | 1.1940(20) | 35 |
| Vinyl Crotonate |
CH3CH=CHCOO-CH=CH2 |
14861-06-4 | -60 | 132.7/760 | 32.0 | 0.9410(20) |
| Vinyl Propionate | CH3CH2COO-CH=CH2 | 105-38-4 | -80 | 90/760 | 4.5 | 0.9170(20) | |
| Vinyl Butyrate | CH3(CH2)2COO-CH=CH2 | 123-20-6 | -80 | 116.7/760 | 19.5 | 0.9002(20) | -5 |
| Vinyl Caproate | CH3(CH2)4COO-CH=CH2 | 3050-69-9 | -52 | 166/760 | 51.0 | 0.8870(20) | -20 |
| Vinyl Caprylate | CH3(CH2)6COO-CH=CH2 | 818-44-0 | -31 | 90/15 | 75.0 | 0.8803(20) | |
| Vinyl Caprate | CH3(CH2)8COO-CH=CH2 | 4704-31-8 | -9.5 | 148/50 | 106 | 0.8752(20) | -60 |
| Vinyl Laurate | CH3(CH2)10COO-CH=CH2 | 2146-71-6 | 6 | 123/4 | 136 | 0.8721(20) | -75 |
| Vinyl Palmitate | CH3(CH2)14COO-CH=CH2 | 693-38-9 | 26 | 165/2 | 176 | 0.8609(30) | |
| Vinyl 2-Ethylhexanoate |
CH3(CH2)3(C2H5)CHCOO-CH=CH2 |
94-04-2 | -70 | 65/15 | 65.0 | 0.8728(20) | |
| Vinyl Cinnamate | C6H5CH=CHCOO-CH=CH2 | 3098-92-8 | -28 | 125/7 | 132 | 1.0715(20) |

